what happens to a given mass of water as it is heated from 2oc to 4oc?
Specific Gravity (= Relative Density) - SG - is a dimensionless unit defined as the ratio of the density of a substance to the density of a reference substance - at a specified temperature and pressure, and can be expressed equally
SG = ρsubstance / ρreference (3)
where
SG = Specific Gravity of the substance
ρsubstance = density of the fluid or substance [kg/chiliad3]
ρreference = density of the reference [kg/miii]
It is virtually common to employ the density of water at iv oC (39oF) as a reference since h2o at this signal has its highest density of 1000 kg/thousand 3 or 1.940sl/ftthree . Still, also the density at 60 °F (xv.6°C) or 20 °C is often used as the reference temperature, e.one thousand. related to crude oils and petroleum products. Water is usually also used as reference when computing the specific gravity for solids. For gases it is air at room temperature (25°C). Pressure is nearly always 1 atm (101.325 kPa). The temperature and pressure must always be specified for both the sample and the reference.
Specific Gravity - SG - is dimensionless and has the same value in the SI system and the Purple English language system (BG).
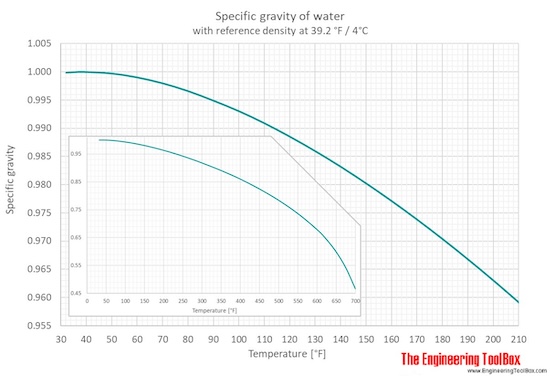
Specific gravity of liquid h2o ranging 32 - 700 oF and 0 - 370 oC is given in figures and tables below:
See H2o and Heavy Water - thermodynamic backdrop.
Run across likewise WaterHumid points at high pressure, Boiling points at vacuum pressure, Density, specific weight and thermal expansion coefficient, Dynamic and kinematic viscosity, Enthalpy and entropy, Heat of vaporization, Ionization Constant, pKwest, of normal and heavy water, Melting points at high pressure, Saturation pressure, Specific heat (heat capacity) and Specific volume for online calculatores, and like figures and tables as shown below.
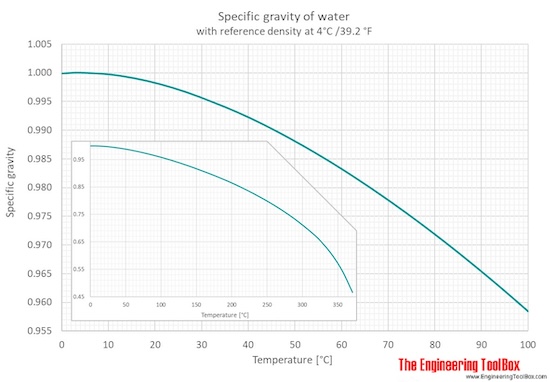
From 0 to 100°C the pressure is i atm, and for temperatures >100°C, the pressure is equal to water saturation pressure.
| Temperature | Pressure | Density | SG at reference temperature | |||
| [°C] | [g/cm3] | 4 °C | 15 °C | 15.6°C ( 60 °F ) | xx °C | |
| 0.1 | 1 atm 14.7 psi 101.3 kPa | 0.999850 | 0.99987 | 1.00075 | one.00083 | 1.00165 |
| 1 | 0.999902 | 0.99993 | 1.00080 | i.00089 | i.00170 | |
| four | 0.999975 | one.00000 | one.00087 | one.00096 | one.00177 | |
| ten | 0.999703 | 0.99973 | one.00060 | 1.00069 | ane.00150 | |
| fifteen | 0.999103 | 0.99913 | 1.00000 | 1.00009 | i.00090 | |
| 20 | 0.998207 | 0.99823 | 0.99910 | 0.99919 | 1.00000 | |
| 25 | 0.997047 | 0.99707 | 0.99794 | 0.99803 | 0.99884 | |
| thirty | 0.995649 | 0.99567 | 0.99654 | 0.99663 | 0.99744 | |
| 35 | 0.99403 | 0.9941 | 0.9949 | 0.9950 | 0.9958 | |
| 40 | 0.99222 | 0.9922 | 0.9931 | 0.9932 | 0.9940 | |
| 45 | 0.99021 | 0.9902 | 0.9911 | 0.9912 | 0.9920 | |
| 50 | 0.98804 | 0.9881 | 0.9889 | 0.9890 | 0.9898 | |
| 55 | 0.98569 | 0.9857 | 0.9866 | 0.9867 | 0.9875 | |
| 60 | 0.98320 | 0.9832 | 0.9841 | 0.9842 | 0.9850 | |
| 65 | 0.98055 | 0.9806 | 0.9814 | 0.9815 | 0.9823 | |
| 70 | 0.97776 | 0.9778 | 0.9786 | 0.9787 | 0.9795 | |
| 75 | 0.97484 | 0.9749 | 0.9757 | 0.9758 | 0.9766 | |
| 80 | 0.97179 | 0.9718 | 0.9727 | 0.9727 | 0.9735 | |
| 85 | 0.96861 | 0.9686 | 0.9695 | 0.9696 | 0.9704 | |
| 90 | 0.96531 | 0.9653 | 0.9662 | 0.9663 | 0.9670 | |
| 95 | 0.96189 | 0.9619 | 0.9628 | 0.9628 | 0.9636 | |
| 99 | 0.95907 | 0.9591 | 0.9599 | 0.9600 | 0.9608 | |
| 99.974 | 0.95837 | 0.9584 | 0.9592 | 0.9593 | 0.9601 | |
| [°C] | Pressure = saturation pressure level [kPa] | [yard/cmiii] | four °C | 15 °C | 15.6°C ( 60 °F ) | 20 °C |
| 100 | 101.4 | 0.9584 | 0.9584 | 0.9592 | 0.9593 | 0.9601 |
| 110 | 143.4 | 0.9510 | 0.9510 | 0.9518 | 0.9519 | 0.9527 |
| 120 | 198.nine | 0.9431 | 0.9431 | 0.9440 | 0.9440 | 0.9448 |
| 140 | 362.3 | 0.9261 | 0.9262 | 0.9270 | 0.9270 | 0.9278 |
| 160 | 620.1 | 0.9075 | 0.9075 | 0.9083 | 0.9083 | 0.9091 |
| 180 | 1003 | 0.8870 | 0.8870 | 0.8878 | 0.8879 | 0.8886 |
| 200 | 1553 | 0.8647 | 0.8647 | 0.8654 | 0.8655 | 0.8662 |
| 220 | 2318 | 0.8402 | 0.8402 | 0.8410 | 0.8410 | 0.8417 |
| 240 | 3347 | 0.8134 | 0.8134 | 0.8141 | 0.8142 | 0.8148 |
| 260 | 4694 | 0.7836 | 0.7836 | 0.7843 | 0.7844 | 0.7850 |
| 280 | 6419 | 0.7503 | 0.7503 | 0.7510 | 0.7510 | 0.7516 |
| 300 | 8588 | 0.7121 | 0.7122 | 0.7128 | 0.7128 | 0.7134 |
| 320 | 11281 | 0.6671 | 0.6671 | 0.6677 | 0.6677 | 0.6683 |
| 340 | 14600 | 0.6107 | 0.6107 | 0.6112 | 0.6113 | 0.6118 |
| 360 | 18672 | 0.5276 | 0.5276 | 0.5281 | 0.5281 | 0.5285 |
| 374 | 22043 | 0.322 | 0.322 | 0.322 | 0.322 | 0.323 |
Specific gravity (SG) for h2o is given for four different reference temperatures (39.two, 59, 60 and 68°F).
From 32 to 212°F the pressure is fourteen.seven psi, and for temperatures >212°F, the force per unit area is equal to water saturation pressure level.
| Temperature | Force per unit area | Density | SG at refence temperatures | |||
| [°F] | [lbm/ft3] | 39.2°F | 59°F ( 15°C ) | 60°F | 68°F ( 20°C ) | |
| 32.2 | 14.7 psi 101.3 kPa 1 atm | 62.418 | 0.9999 | 1.0007 | 1.0008 | ane.0016 |
| 34 | 62.422 | 0.9999 | 1.0008 | 1.0009 | 1.0017 | |
| 39.2 | 62.426 | 1.0000 | 1.0009 | one.0010 | one.0018 | |
| 50 | 62.409 | 0.9997 | 1.0006 | 1.0007 | 1.0015 | |
| threescore | 62.366 | 0.9990 | 0.9999 | i.0000 | 1.0008 | |
| 70 | 62.301 | 0.9980 | 0.9989 | 0.9990 | 0.9998 | |
| 80 | 62.215 | 0.9966 | 0.9975 | 0.9976 | 0.9984 | |
| xc | 62.109 | 0.9949 | 0.9958 | 0.9959 | 0.9967 | |
| 100 | 61.993 | 0.9931 | 0.9939 | 0.9940 | 0.9948 | |
| 110 | 61.860 | 0.9909 | 0.9918 | 0.9919 | 0.9927 | |
| 120 | 61.712 | 0.9886 | 0.9894 | 0.9895 | 0.9903 | |
| 130 | 61.551 | 0.9860 | 0.9868 | 0.9869 | 0.9877 | |
| 140 | 61.379 | 0.9832 | 0.9841 | 0.9842 | 0.9850 | |
| 150 | 61.194 | 0.9803 | 0.9811 | 0.9812 | 0.9820 | |
| 160 | 61.000 | 0.9772 | 0.9780 | 0.9781 | 0.9789 | |
| 170 | sixty.795 | 0.9739 | 0.9747 | 0.9748 | 0.9756 | |
| 180 | 60.579 | 0.9704 | 0.9713 | 0.9713 | 0.9721 | |
| 190 | lx.354 | 0.9668 | 0.9677 | 0.9677 | 0.9685 | |
| 200 | 60.121 | 0.9631 | 0.9639 | 0.9640 | 0.9648 | |
| 210 | 59.878 | 0.9592 | 0.9600 | 0.9601 | 0.9609 | |
| 211.95 | 59.829 | 0.9584 | 0.9592 | 0.9593 | 0.9601 | |
| [°F] | Pressure = saturation force per unit area [psi] | [lbchiliad/ft3] | 39.2°F | 59°F ( 15°C ) | 60°F | 68°F ( 20°C ) |
| 220 | 17.ii | 59.63 | 0.9552 | 0.9560 | 0.9561 | 0.9569 |
| 240 | 25.0 | 59.10 | 0.9467 | 0.9475 | 0.9476 | 0.9484 |
| 260 | 35.5 | 58.53 | 0.9375 | 0.9384 | 0.9384 | 0.9392 |
| 280 | 49.3 | 57.93 | 0.9279 | 0.9287 | 0.9288 | 0.9296 |
| 300 | 67.three | 57.29 | 0.9177 | 0.9185 | 0.9186 | 0.9193 |
| 350 | 135 | 55.59 | 0.8905 | 0.8913 | 0.8914 | 0.8921 |
| 400 | 247 | 53.67 | 0.8598 | 0.8606 | 0.8606 | 0.8613 |
| 450 | 422 | 51.45 | 0.8242 | 0.8250 | 0.8250 | 0.8257 |
| 500 | 681 | 48.92 | 0.7837 | 0.7843 | 0.7844 | 0.7850 |
| 550 | 1045 | 45.95 | 0.7360 | 0.7367 | 0.7367 | 0.7373 |
| 600 | 1542 | 42.36 | 0.6786 | 0.6792 | 0.6793 | 0.6798 |
| 625 | 1851 | twoscore.12 | 0.6426 | 0.6432 | 0.6433 | 0.6438 |
| 650 | 2208 | 37.35 | 0.5982 | 0.5988 | 0.5988 | 0.5993 |
| 675 | 2619 | 33.79 | 0.5412 | 0.5417 | 0.5417 | 0.5422 |
| 700 | 3092 | 29.07 | 0.4657 | 0.4661 | 0.4662 | 0.4666 |
Source: https://www.engineeringtoolbox.com/water-temperature-specific-gravity-d_1179.html
0 Response to "what happens to a given mass of water as it is heated from 2oc to 4oc?"
Post a Comment